Background: Cytomegalovirus (CMV) infection is a major cause of morbidity and mortality after hematopoietic stem cell transplantation (HSCT). It causes end-organ disease, multi-organ dysfunction syndrome, graft failure, increased susceptibility to infections and GVHD. According to the published western data greatest risk of CMV infection is the seropositivity of the recipient, however, in a high endemic population where seropositivity is up to 100%, risk factors for CMV reactivation are different and are analyzed in this study.
Methods: It is a prospective descriptive study performed at Armed Forces Bone Marrow Transplant Center, Rawalpindi, Pakistan from January 2017 to March 2020. Consecutive patients who underwent allogeneic HSCT during this period were enrolled. All patients were prospectively monitored for CMV reactivation by weekly or two weekly CMV DNA quantitative PCR, from engraftment till day 100 post-transplant. CMV infection was diagnosed on detection of more than 200 copies/ml on PCR. Threshold for starting preemptive antiviral therapy was kept at 2000 copies/ml. Patients with past history of CMV infection, those who expired before day 14 post-transplant or those with less than 70% of required CMV tests were not included in the study. Factors associated with CMV reactivation, outcome of antiviral therapy and effect of CMV on post-transplant survival were studied.
Results: Out of 319 transplants during this period, 230 patients fulfilled the inclusion criteria. Of these, 197 were HLA matched sibling, 18 were matched family donor and 15 were haploidentical transplants. There were 163 males and 67 females. Median age at transplant was 9.5 years (0.5-53). Eighty-three transplants were done in thalassemia, 55 in aplastic anemia, 14 in Fanconi anemia, 27 in acute leukemias, 8 in CML, 9 in MDS, 12 in HLH and 22 in other hematological disorders. All the patients and donor were CMV IgG seropositive when tested before transplantation.
CMV reactivation was seen in 152 out of 230 patients (66.1%). Of 152, 95 patients had CMV viral load more than 2000 copies/ml and required antiviral treatment. Median time to reactivation since transplant was 35 days (13-90).
In multivariate analysis using binary regression, risk factors for high viral load CMV reactivation included steroid administration (p=0.009), recipient age less than 10 years (p=0.003) and haploidentical transplant (p=0.048). No statistically significant association was found with the use of ATG, GVHD, underlying disease, ABO blood group or gender mismatch.
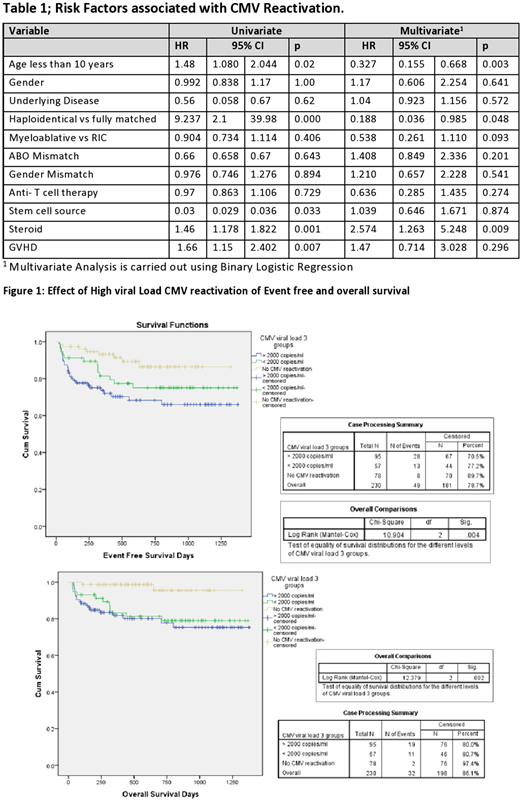
Survival analysis using cox regression showed significant impact of high viral load CMV reactivation on post-transplant survival. Event-free survival (EFS) with and without CMV reactivation was 70.5 % and 89.7% respectively (p=0.004) and overall survival (OS) was 80.0 % and 97.4 % with and without CMV reactivation respectively (p=0.002).
Valganciclovir was given in 89 patients and 6patients were treated with ganciclovir. Mean time to clear viremia was 19.8±9 days. Myelosuppression was seen in 41% of patients treated with valganciclovir. Renal impairment was seen in 25% of patients treated with valganciclovir. One patient had resistant disease. One patient had CMV pneumonia and she recovered. One patient died of suspected CMV pneumonia
Conclusion: CMV reactivation was seen in 66.1% of the transplant recipients, this is higher compared to the western world due to high CMV seropositivity is this region. Steroids administration in post-transplant period significantly increase the risk of CMV reactivation. Preemptive therapy with valganciclovir effectively treats CMV reactivation. Viral threshold for treatment should be decided considering the regional endemicity. CMV adversely effects the transplant outcome in terms of EFS and OS.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.